
October 28, 2024
Valisure’s Grand Opening of our New State-Of-The-Art Laboratory
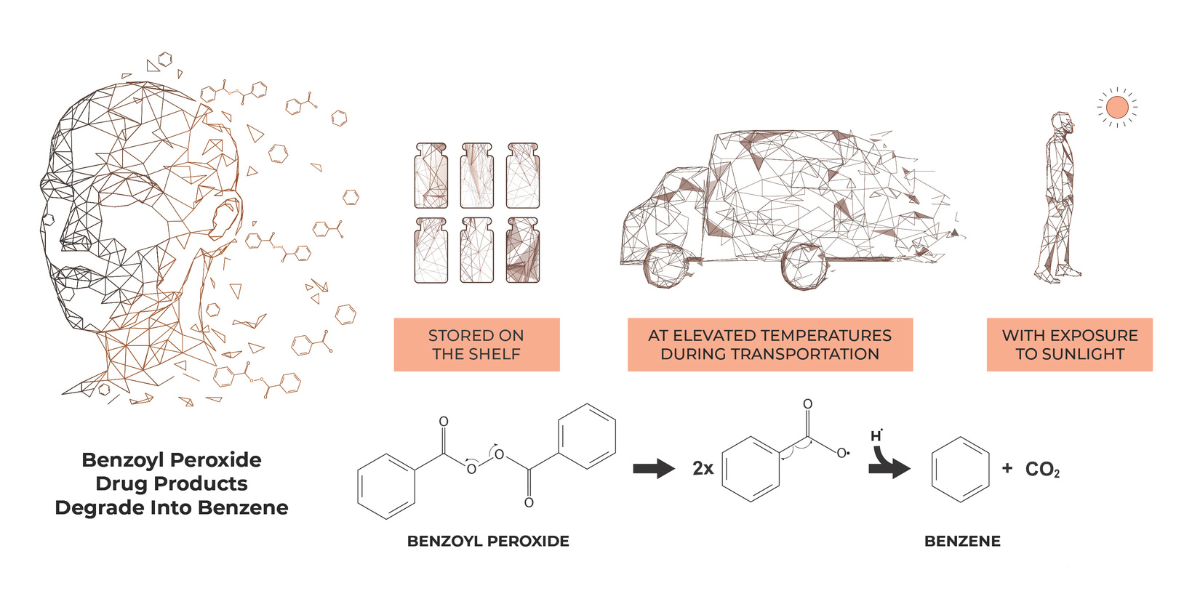
October 7, 2024
New Valisure Scientific Paper on Benzene in Benzoyl Peroxide Products

March 7, 2024
What is "Stability Testing" & Why it's Important

March 6, 2024
Valisure Detects Benzene in Benzoyl Peroxide

February 28, 2024
Hartford Business Journal | Valisure Announces New CEO & Investments

January 1, 2023
GOJO & Valisure: Promoting Public Health Through Collaboration

November 1, 2022
Valisure Detects Benzene in Dry Shampoo

November 1, 2022
Timeline of Consumer Product Recalls Due to Benzene

November 4, 2021
Valisure Detects Benzene in Body Spray

March 6, 2024
FDA Citizen Petition #8: Benzene in Benzoyl Peroxide Products

March 7, 2023
Valisure’s FDA Citizen Petitions

November 1, 2022
FDA Citizen Petition #7: Benzene in Dry Shampoo Products

November 4, 2021
FDA Citizen Petition #6: Benzene in Body Spray Products

May 25, 2021
FDA Citizen Petition #5: Benzene in Sunscreen Products

March 24, 2021
FDA Citizen Petition #4: Benzene in Hand Sanitizer Products

March 2, 2020
FDA Citizen Petition #3: NDMA Carcinogen in Metformin

September 13, 2019
FDA Citizen Petition #2: NDMA Carcinogen in Ranitidine (Zantac) & Nizatidine (Axid)

June 13, 2019
FDA Citizen Petition #1: DMF Carcinogen in Valsartan

March 14, 2024
EHP: Benzoyl Peroxide Drug Products Form Benzene

October 31, 2023
AJMC: Safety vs Price in the Generic Drug Market: Metformin

April 26, 2023
Valisure Presentation at Stanford Medicine Grand Rounds

October 10, 2022
JAPhA: A Data-Driven Quality-Score System

June 3, 2022
Journal of Pharmaceutical Innovation: FDA Approaches in Monitoring Drug Quality

April 18, 2022
Circulation: Price and Quality in the Generic Pharmaceutical Market

April 15, 2022
JDD: Dermatologists’ Responses to Benzene Being Reported as a Contaminant in Sunscreen: A Cross-Sectional Analysis

March 29, 2022
Environmental Health Perspectives: Independent Sun Care Product Screening for Benzene Contamination

March 18, 2022
A Joint Study on Hand Sanitizer Published in the Public Library Of Science

September 9, 2020
Katherine Eban on Valisure Video Seminar

February 3, 2020
Valisure Participation in Duke Margolis Center Event: Understanding How the Public Perceives and Values Pharmaceutical Quality

March 23, 2022
The New York Times | Pfizer Recalls Some Blood Pressure Drugs, Citing Cancer Risk

March 18, 2022
The Guardian | Carcinogenic Chemical Benzene Found in Hundreds of US Personal Care Products

December 29, 2021
Bloomberg | Toxins in Household Products Leave FDA Chasing a Vapor Trail

December 20, 2021
C&EN | Finding Benzene Everywhere We Look

November 4, 2021
HBW Insight | Valisure’s Findings Of Benzene In Body Sprays Suggest Ongoing Aerosol Propellant Problem

November 1, 2021
New Haven Biz | Valisure Capitalizes On Growing Demand For Drug Quality Checks

September 18, 2021
The New York Times | Our Drug Supply Is Sick. How Can We Fix It?

May 26, 2021
Dermatology Times | Valisure Highlights Controversy of Benzene Contamination in Sunscreen Products

May 26, 2021
HBW Insight | Valisure’s Benzene Testing Finds Problems With OTC Sunscreens, Cosmetic Sun Care Products

May 3, 2022
Valisure Named Honorable Mention in Category of Fast Company’s 2022 World Changing Ideas Awards

April 12, 2022
David Light Awarded Junior Achievement's Entrepreneur of The Year Award

March 10, 2022
Valisure First Company To Be Named By MedPage As A Healthcare Disruptor

November 1, 2019
U.S. Senator Chris Murphy Honors Valisure With His Office’s Innovator Award

April 26, 2018
Valisure Wins Pre-Revenue Venture Of The Year In 2018 CT Entrepreneur Awards

November 14, 2022
Congresswoman Dingell & Congresswoman Stevens Letter To FDA

June 2, 2021
Valisure Testimony at Senate Finance Committee

May 28, 2021
U.S. Senator Blumenthal’s Reaction to Benzene in Sunscreens

December 18, 2019
Congresswoman DeLauro Letter to FDA On Ranitidine

February 16, 2017
Congresswoman Rosa DeLauro and The Recall Unsafe Drugs Act















































