
May 25, 2021
Valisure Detects Benzene in Sunscreen

March 24, 2021
Valisure Detects Benzene in Hand Sanitizer

March 2, 2020
Valisure Detects NDMA in Metformin

September 13, 2019
Valisure Detects NDMA in Ranitidine

June 13, 2019
Valisure Detects DMF in Valsartan

Responsible Disposal of Contaminated Products

March 7, 2024
A Track Record of Action: Valisure’s FDA Citizen Petitions Driving Industry-Wide Change

March 6, 2024
FDA Citizen Petition #8: Benzene in Benzoyl Peroxide Products

November 1, 2022
FDA Citizen Petition #7: Benzene in Dry Shampoo Products

November 4, 2021
FDA Citizen Petition #6: Benzene in Body Spray Products

May 25, 2021
FDA Citizen Petition #5: Benzene in Sunscreen Products

March 24, 2021
FDA Citizen Petition #4: Benzene in Hand Sanitizer Products

March 2, 2020
FDA Citizen Petition #3: NDMA Carcinogen in Metformin

September 13, 2019
FDA Citizen Petition #2: NDMA Carcinogen in Ranitidine (Zantac) & Nizatidine (Axid)

June 13, 2019
FDA Citizen Petition #1: DMF Carcinogen in Valsartan
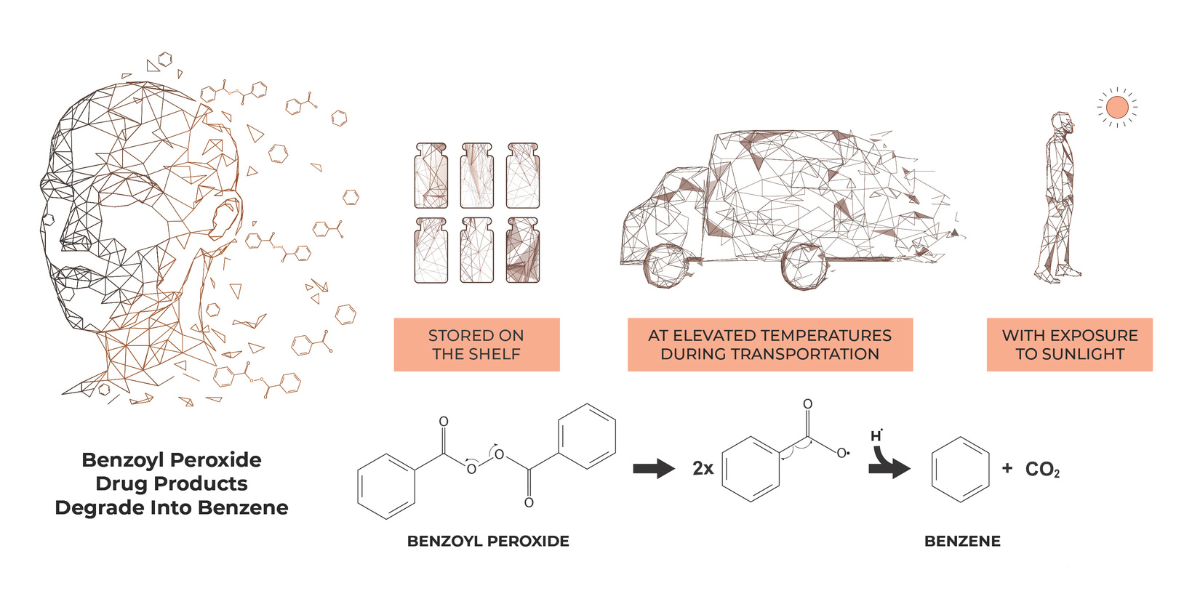
October 7, 2024
New Valisure Scientific Paper on Benzene in Benzoyl Peroxide Products

March 14, 2024
Environmental Health Perspectives Publishes Study on Benzene Formation in Benzoyl Peroxide Products

October 31, 2023
AJMC: Safety vs Price in the Generic Drug Market: Metformin

April 26, 2023
Valisure Presentation at Stanford Medicine Grand Rounds

October 10, 2022
JAPhA: A Data-Driven Quality-Score System

June 3, 2022
Journal of Pharmaceutical Innovation: FDA Approaches in Monitoring Drug Quality

April 18, 2022
Circulation: Price and Quality in the Generic Pharmaceutical Market

April 15, 2022
JDD: Dermatologists’ Responses to Benzene Being Reported as a Contaminant in Sunscreen: A Cross-Sectional Analysis

March 29, 2022
Environmental Health Perspectives: Independent Sun Care Product Screening for Benzene Contamination

April 9, 2025
Valisure Joins HCMA Legislative Briefing to Advance Drug Quality Reform

September 25, 2024
AHRMM24 DoD-led Pharmaceutical Quality Assessment

September 19, 2024
Fireside Chat: National Security & the Pharmaceutical Supply Chain

September 13, 2024
Valisure Presents Initial Findings from Department of Defense Drug Quality Study

March 5, 2024
Vote & Participate in Nationwide Independent Pharmaceutical Quality Study

September 12, 2023
Health Affairs | Ensuring Access To Generic Medications In The US

August 23, 2023
Webinar: Quantitative Impurities Analysis of Consumer Products using SIFT-MS

August 7, 2023
Health Affairs | How To Ensure The Quality And Safety Of Our Generic Drugs

August 6, 2023
AHRMM: A Quadruple Threat to Public Health & National Security

October 9, 2019
CBS News | Potentially Dangerous Chemical Found in Popular Heartburn Pill Zantac

October 2, 2019
Bloomberg | Leading Cancer Center No Longer Offering Zantac to Patients

September 12, 2019
Bloomberg Business Week | Can You Trust Generics?

November 14, 2018
The Washington Post | ‘Rapid release’ Tylenol gelcaps are slower to dissolve than cheaper tablets, study finds

May 3, 2022
Valisure Named Honorable Mention in Category of Fast Company’s 2022 World Changing Ideas Awards

April 12, 2022
David Light Awarded Junior Achievement's Entrepreneur of The Year Award

March 10, 2022
Valisure First Company To Be Named By MedPage As A Healthcare Disruptor

November 1, 2019
U.S. Senator Chris Murphy Honors Valisure With His Office’s Innovator Award

April 26, 2018
Valisure Wins Pre-Revenue Venture Of The Year In 2018 CT Entrepreneur Awards

April 3, 2025
Valisure Presents DoD Drug Quality Findings at State of the Science Symposium 2025

October 31, 2024
Senator Richard Blumenthal: A Champion for Medication and Consumer Product Safety

October 31, 2024
Joint Press Conference: Blumenthal & Valisure Address Benzene in Acne Products

October 28, 2024
A Shared Commitment to Drug Safety: Congresswoman Rosa DeLauro and Valisure

June 3, 2024
Valisure and FDA Leadership Meeting

April 30, 2024
Senate Finance Committee Hearing: Advancing Medication Quality for the Military

April 2, 2024
Valisure Presents at Uniformed Services University

December 29, 2023
FDA New December 2023 Guidance

August 8, 2023
Valisure & DoD Sign Agreement For Testing Drug Quality


















































